
| Chungbuk National University Researchers Develop a Novel Electrocatalyst for Efficient Hydrogen Production | |||||||
| Writer | 관리자 | ||||||
|---|---|---|---|---|---|---|---|
Chungbuk National University Researchers Develop a Novel Electrocatalyst for Efficient Hydrogen Production
The new nanostructured nickel cobalt molybdate hydrate catalyst has an enhanced oxygen evolution reaction performance and can be used to generate hydrogen from water
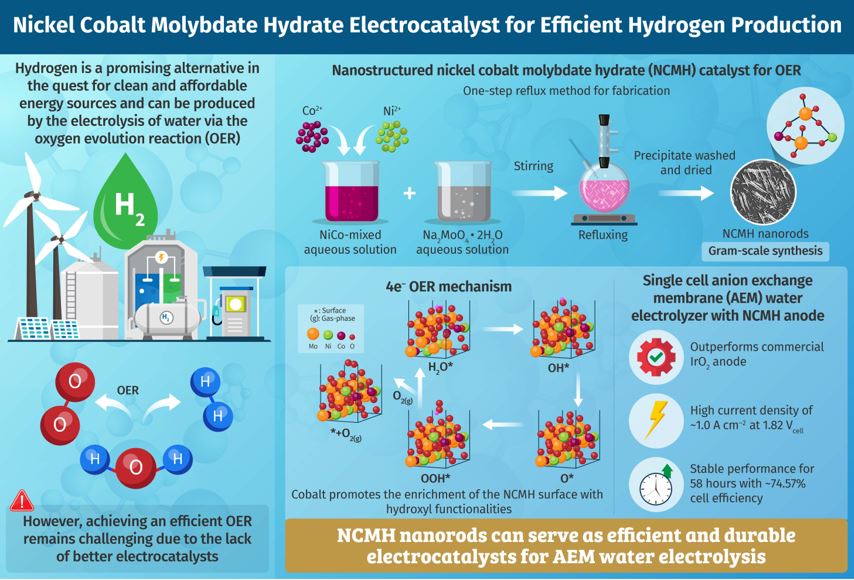
As fossil fuel sources dwindle and the environmental concerns stemming from their use grow, searching for clean and affordable energy sources is crucial. Hydrogen is a promising candidate, but its production relies on rare and costly catalysts. To tackle this, researchers have recently fabricated a highly efficient nickel cobalt molybdate hydrate electrocatalyst, using a simple and scalable method. Its outstanding performance makes this catalyst a game-changer in the sustainable production of hydrogen. Amidst the looming depletion of fossil fuels and the urgent imperative to combat environmental pollution, the pursuit of clean and cost-effective energy sources is of paramount importance. Hydrogen is a promising clean energy source, and water electrolysis is a green way to produce it. However, efficiently splitting water into hydrogen and oxygen remains a complex task.
The key to overcoming this challenge lies in the development of efficient electrocatalysts. While materials based on noble metals such as ruthenium and iridium are excellent candidates, their widespread use is hindered by their high costs and limited availability. Recently, transition metal molybdates, especially those engineered into nanostructures, have emerged as a potential alternative. However, the complex and environmentally harmful production processes associated with these materials pose a major challenge.
To tackle this problem, a team of researchers, led by Professor Sang Mun Jeong and including Professor Dong-Hee Lim and Assistant Professor Yoo Sei Park, all from Chungbuk National University, has recently synthesized nickel cobalt molybdate hydrate (NCMH) nanostructured rods on gram scale using a simple one-step reflux method. The electrocatalyst was fabricated by mixing solutions of nickel, cobalt, and molybdenum salts and applying heat.
Their work was made available online on 20 February 2023 and published in Volume 328 of the journal Applied Catalysis B: Environmental on 5 July 2023.
The researchers explored the electrocatalytic performance of NCMH in the oxygen evolution reaction (OER, a crucial step of water decomposition), theoretically as well as experimentally. They utilized density functional theory, a quantum mechanics-based computational chemistry modeling method, to elucidate the OER pathway.
Prof. Jeong explains: “The combination of nickel and cobalt turned out to be crucial for NCMH's ability to work as a catalyst. When we compare this material with nickel molybdate hydrate, it becomes evident that the addition of cobalt makes a substantial difference in enhancing NCMH’s capacity to facilitate efficient electrocatalytic performance.” Cobalt promotes the adsorption of hydroxyl groups, enriching the surface of NCMH with hydroxyl functionalities, which, in turn, improves its interfacial electrochemistry. Consequently, NCMH exhibits excellent performance in OER.
The team conducted several electrochemical tests to demonstrate the novelty of their proposed catalyst. They found that a single cell anion exchange membrane (AEM) water electrolyzer containing an NCMH anode exhibited superior performance compared to an electrolyzer with a commercial iridium (IV) oxide anode. The former had a high current density of ~1.0 A cm–2 at 1.82 Vcell, a low overpotential of ~291 mV at 10 mA cm–2, and a remarkable long-term stability of 58 hours with ~74.57% cell efficiency. Therefore, NCMH nanorods are promising anode materials for AEM water electrolysis, which can lead to efficient hydrogen production.
Prof. Lim highlights the future implications of their work. “The hydrogen produced through water decomposition is utilized in advanced fuel cell technologies that can generate electricity without emitting pollutants. This environmentally friendly electricity generation, free from fossil fuel use, is expected to contribute toward carbon neutrality.”
“Moreover, it will enable cost-effective high-energy utilization in various sectors, providing a solution for a wide range of energy needs,” concludes Dr. Park.
Let us hope that this technology leads to many more hydrogen-powered innovations and power plants!
Reference
About the institute
Chungbuk National University, located in Cheongju, South Korea, is a distinguished institution renowned for its commitment to academic excellence, research innovation, and community engagement. Founded in 1951, the university offers a wide spectrum of undergraduate, graduate, and doctoral programs spanning diverse fields and maintaining rigorous academic standards. It is a hub of cutting-edge research, with numerous research centers and institutes dedicated to technological advancements, healthcare, agriculture, and cultural studies.
About the authors
Prof. Sang Mun Jeong is a Professor in the Department of Chemical Engineering at Chungbuk National University, a leader of the Regional Leading Research Center (RLRC) for developing next-generation battery materials funded by National Research Foundation of Korea, and a Director of Korea Institute of Chemical Engineers. Also, he was Dean of Research Affairs at Chungbuk National University (2021–2023). His research focuses on energy-related materials and processes based on chemical and electrochemical engineering to develop efficient, clean, and renewable future energy.
Prof. Dong-Hee Lim is a Professor in the Department of Environmental Engineering at Chungbuk National University. His research group is dedicated to elucidating various chemical reaction mechanisms using quantum chemistry-based modeling. He earned his Ph.D. degree from the University of Michigan-Ann Arbor (USA) and subsequently completed a postdoctoral fellowship at Stanford University (USA).
Prof. Yoo Sei Park is an Assistant Professor in the Department of Advanced Material Engineering at Chungbuk National University. His research interests include AEM electrolyzers, electrode manufacturing, water electrolysis, and proton conducting fuel cells. He has published around 40 research papers, which have been cited over 800 times. |
|||||||
| Attachments | |||||||